Ferguson Lab
Thomas Ferguson, PhD, Professor, Ophthalmology and Visual Sciences

Research
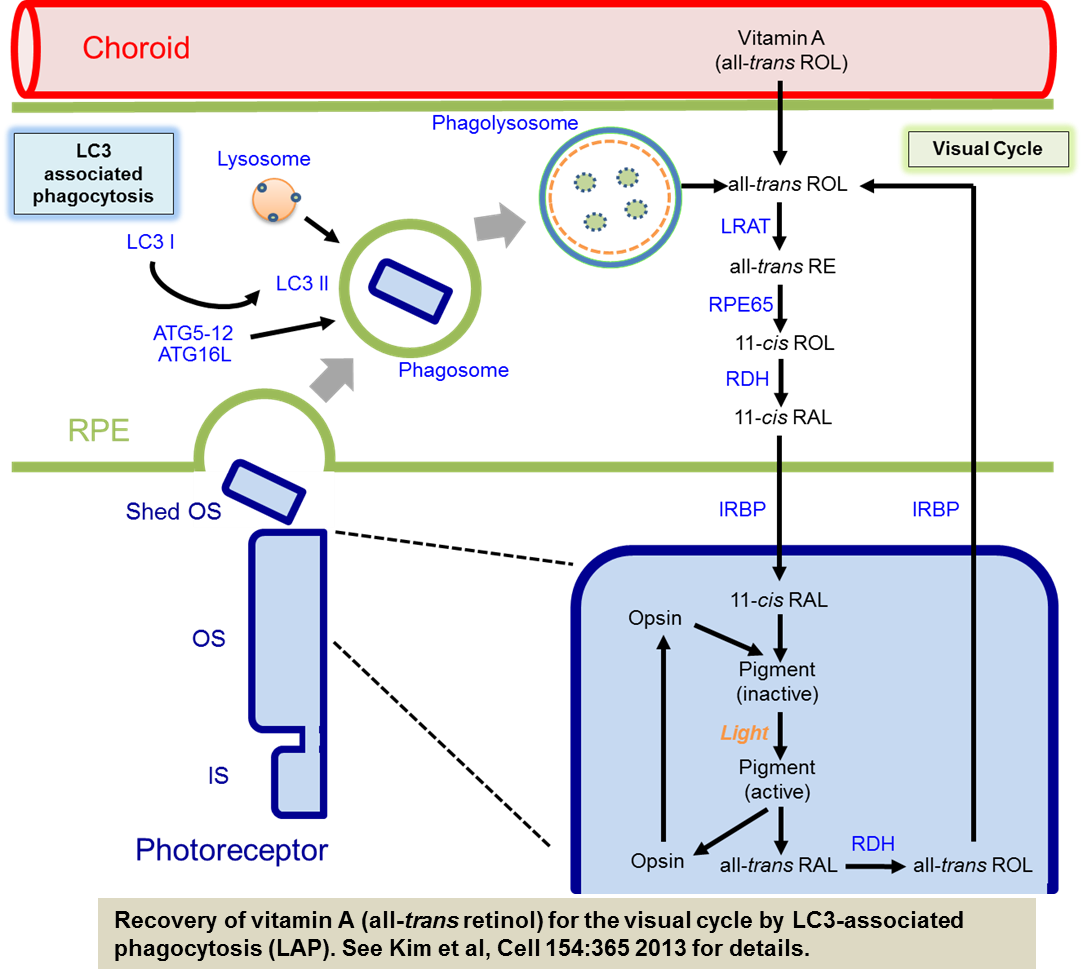
The laboratory studies the role of autophagy in the pathogenesis of eye diseases such as age-related macular degeneration (AMD) and other retinal degenerative diseases. We are pursuing this by creating mice in which basal autophagy is deleted in specific cell types in the eye. Recently we found that a link between the autophagy machinery, phagocytosis of photoreceptor outer segments, and the regeneration of chromophore by the visual cycle in the retinal pigment epithelium (RPE). Our results showed that the autophagy protein LC3 associates with single membrane phagosomes containing engulfed POS in an Atg5-dependent manner that also requires Beclin1 but not Ulk1, Atg13, or Fip200 of the autophagy pre-initiation complex. This process termed LC3 associated phagocytosis (LAP) is necessary for optimal vision. Thus, in contrast to conventional autophagy, our studies suggest that a non-canonical form of autophagy functions to support chromophore regeneration through the efficient processing of outer segments by the RPE.
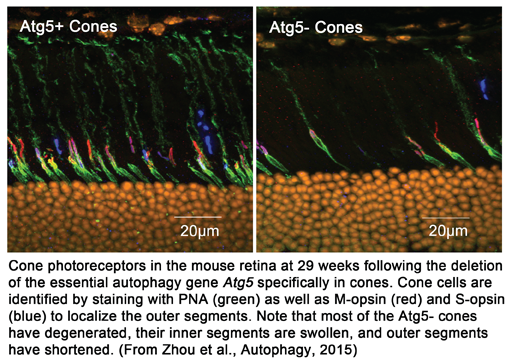
Our studies have also shown that autophagy is critical to function and long term survival of rod and cone photoreceptors. Studying autophagy in photoreceptors has given us new insights into the metabolism, function, and stress responses of these cells that are critical to vision. Cones are particularly dependent on autophagy as they utilize this process for their continued function in intense bright light and activate the pathway in response to light stress and systemic nutrient starvation.
Although we are members of the vision science community at Washington University we are also part of the Immunology group. Our studies have concentrated on the molecular basis of immune tolerance and how apoptotic cells tolerize the immune response. Our studies try to understand the molecular basis of this type of immune regulation using biochemistry and gene targeting technology. Currently we are studying what it is about apoptosis, per se, that reprograms dendritic cells away from their ability to activate immunity to potent inducers of immune tolerance. We have determined that during apoptosis the activation of caspases and the destruction of the mitochondria are critical in the ability of apoptotic cells to induce immune tolerance. A key feature is that the production of ROS (reactive oxygen species) during apoptosis oxidizes (and inactivates) the damage associated molecule HMGB1 high mobility group box 1 protein). When HMGB1 is not oxidized, apoptotic cells can stimulate the immune response. Thus one role of the apoptotic process is the inactivation of damage associated signals to prevent aberrant immune responses.

Neovascularization is a major component of blinding ocular disorders such as AMD. While there are 2 general types of AMD (dry and wet) most of the blindness is the result of neovascular AMD (wet form) where choroidal neovascularization beneath the retinal leads to irreversible blindness in the elderly. Currently therapies can slow the progression of AMD but do little to reverse the eventual blindness in most patients. Recent data suggest that chronic inflammation may be an important component of neovascular AMD. Studies into the inflammatory nature of this disease often overlook the immune privileges status of the eye. The mechanisms of immune privilege function to limit inflammation at the expense of certain immune effector mechanisms as even minor bouts of inflammation can have devastating consequences for delicate structures such as the retina. An important part of immune privilege is the constitutive expression of the death-inducing protein, FasL, which inhibits inflammation and neovascularization by inducing apoptosis in cells expressing the Fas receptor. Our studies in examine the hypothesis that modulating immune privilege, specifically FasL, may have beneficial effects in neovascular eye disease. In addition, we explore the effects of aging on the expression and function of this important pro-apoptotic protein. Examining neovascular eye disease from the perspective of immune privilege will give important new insights into pathogenesis and treatment of blinding eye disorders. In addition we are using several mouse models of AMD to explore the role of immune privilege in this disease.
Age-related macular degeneration (AMD) is the leading cause of blindness in industrialized countries. In spite of recent advances in our understanding of AMD, treatments are limited. Evidence suggests that dysfunction in the retinal pigment epithelial (RPE) layer plays a central role in the development of the disease. Autophagy is a conserved intracellular process responsible for the continuous clearance of cellular components. This process is critical to cell survival by clearing damaged proteins and mitochondria in the lysosomes. Additionally, it provides a survival pathway when nutrients are limiting and under conditions of oxidative stress. Importantly, it has been established that autophagy declines with age. Since aging results in the accumulation of oxidative products and damaged mitochondria in the RPE, it is reasonable to assume that changes in autophagy may be responsible for some of the observed age-related dysfunctions that can lead to eye disease; however, this has not been tested. The goal of our current studies is to explore the role of autophagy in eye disease by examining the consequences of the specific deletion of the essential autophagy genes in the eyes of mice.
Using these unique mouse strains we examine age related physiological, morphological and ultrastructural changes in the eye. This includes analysis of rod and cone photoreceptor function, the visual cycle, as well as light and electron microscopic examination of the retina and RPE. Our studies have also shown that autophagy is critical to function and long term survival of rod and cone photoreceptors. Studying autophagy in photoreceptors has given us new insights into the metabolism, function, and stress responses of these cells that are critical to vision. Cones are particularly dependent on autophagy as they utilize this process for their continued function in intense bright light and activate the pathway in response to light stress and systemic nutrient starvation.
Our studies have concentrated on the molecular basis of immune tolerance and how apoptotic cells tolerize the immune response. Our studies try to understand the molecular basis of this type of immune regulation using biochemistry and gene targeting technology. Currently we are studying what it is about apoptosis, per se, that reprograms dendritic cells away from their ability to activate immunity to potent inducers of immune tolerance. We have determined that during apoptosis the activation of caspases and the destruction of the mitochondria are critical in the ability of apoptotic cells to induce immune tolerance. A key feature is that the production of ROS (reactive oxygen species) during apoptosis oxidizes (and inactivates) the damage associated molecule HMGB1 high mobility group box 1 protein). When HMGB1 is not oxidized, apoptotic cells can stimulate the immune response. Thus one role of the apoptotic process is the inactivation of damage associated signals to prevent aberrant immune responses.
Publications
View Thomas Ferguson’s NCBI publications on PubMed»
Recent Original Reports:
- Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV, Tang PH, Ablonczy Z, Chan CC, Zhou Z, Green DR, Ferguson TA. Noncanonical autophagy promotes the visual cycle. Cell 154:365-376. 2013. PMC3744125
- Zhou Z, Doggett TA, Sene A, Apte RS, Ferguson TA. Autophagy supports survival and phototransduction protein levels in rod photoreceptors. Cell Death Differ. 22:488-98, 2015. PMC4326583
- Doyle SL, López FJ, Celkova L, Brennan K, Mulfaul K, Ozaki E, Kenna PF, Kurali E, Hudson N, Doggett T, Ferguson TA, Humphries P, Adamson P, Campbell M. IL-18 Immunotherapy for Neovascular AMD: Tolerability and Efficacy in Nonhuman Primates. Invest Ophthalmol Vis Sci. 56:5424-30, 2015. PMID: 26284546
- Zhou Z, Vinberg F, Schottler F, Doggett TA, Kefalov VJ, Ferguson TA. Autophagy Supports Color Vision. Autophagy, 11:1821-32. 2015 PMC4824586.
- Yin XT, Keadle TL, Hard J, Herndon J, Potter CA, Del Rosso CR, Ferguson TA, Stuart PM. Impaired Fas-Fas Ligand Interactions Result in Greater Recurrent Herpetic Stromal Keratitis in Mice. J Immunol Res. 2015; PMC4609448
- Ferguson TA, Laurie GW. Introduction to Autophagy in the Eye (or “What’s Eatin’ You?”). Exp Eye Res. 2016 PMC4777279
- Santeford A, Wiley LA, Park S, Bamba S, Nakamura R, Gdoura A, Ferguson TA, Rao PK, Guan JL, Saitoh T, Akira S, Xavier R, Virgin HW, Apte RS. Impaired Autophagy in Macrophages Promotes Inflammatory Eye Disease. Autophagy Epub July 2016. PMID: 27463423
Book Chapters
- Ferguson TA and Niederkorn JA: Anterior Chamber Associated Immune Deviation. IN: Ocular Infections and Immunity. JS Pepose, GN Holland, and KR Wilhelmus, eds. Mosby-Year Book, Inc., Philadelphia, PA 1995
- Ferguson TA and Van Gelder, RV: Immune Function and Ocular Disease. IN: Samter’s Immunologic Diseases, Sixth edition. KF Austin, MM Frank, JP Atkinson, and H Cantor, eds. Lippincott, Williams & Wilkins, Baltimore, MD. 2001