Wang Lab
Qian Wang, PhD
Signaling networks in eye development and diseases
The Wang Lab’s research examines the signaling networks that control ocular tissue development and homeostasis, particularly the ocular lens and ocular gland (lacrimal gland), with an emphasis on how these processes contribute to congenital and degenerative eye diseases.
Research Overview
We use mouse models to investigate signaling networks underlying eye development and the mechanisms of ocular diseases. We are particularly interested in two ocular tissues, the ocular lens and the ocular gland (lacrimal gland).
We use a combination of techniques including mouse genetics, imaging, cell/molecular biology techniques, and computational/bioinformatic analysis. We are also actively exploring the collaboration with clinicians to conduct research with human eye specimens.
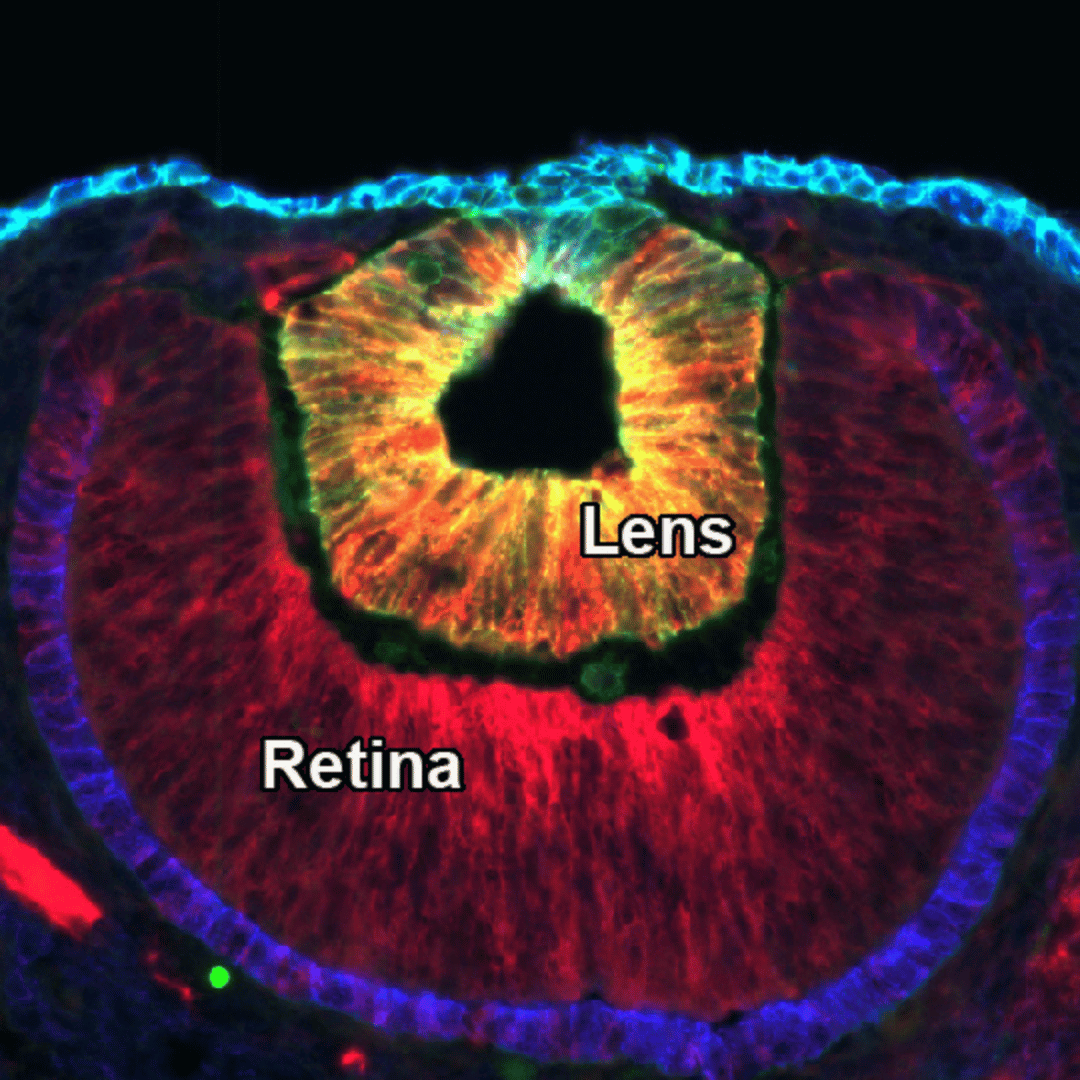
Lens Development
The ocular lens is a transparent structure located at the center of the eye, responsible for focusing light onto the retina to enable clear vision. We are interested in fundamental questions surrounding lens development as well as the mechanisms of lens pathology.
Our research is guided by a fundamental set of questions:
- What signals control lens morphogenesis?
- How do different signaling pathways crosstalk to each other?
- How does lens develop into a transparent structure?
- How does lens maintain its transparency?
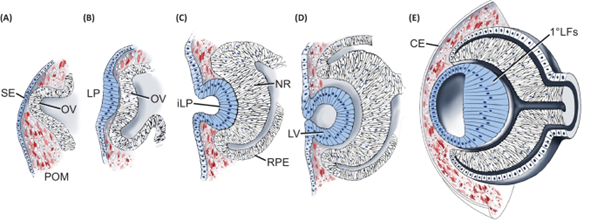
Stages of early lens development in mouse embryos (Cvekl and Zhang, 2017).
Mouse lens development begins with the specification of the lens field (surface ectoderm) at embryonic day E8.5 (A), followed by thickening of the surface ectoderm to form the lens placode at E9.5 (B). At E10.5, the lens placode invaginates to form the lens pit (C), which develops into the lens vesicle by E11.5 (D). Once the lens vesicle separates from the surface ectoderm, the anterior cells maintain their epithelial property while the posterior cells differentiate and elongate into lens fibers (E). Disturbances in these steps can cause lens developmental defects, leading to congenital eye diseases. For examples, failure of lens vesicle separation from the surface ectoderm results in the lens-corneal attachment and formation of persistent lens stalk, a condition known as Peters anomaly in humans. We are currently studying signaling networks that control the lens-corneal separation process.
Lacrimal Gland Development
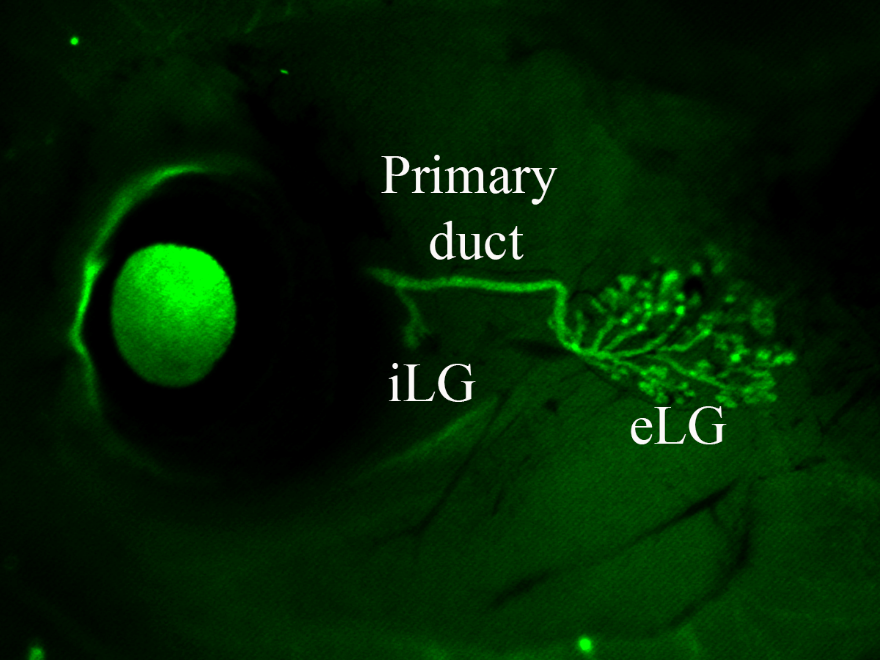
The lacrimal gland is a tear-secreting organ which lubricates and protects the ocular surface. Common conditions related to lacrimal gland dysfunction include dry eye disease, Sjogren’s syndrome (an autoimmune disorder), and dacryoadenitis (inflammation of the lacrimal gland). Understanding lacrimal gland development, function, and pathology is critical for addressing various eye diseases and conditions related to tear production and regulation.
Wang Lab Members

Publications
- Wang Q, Li H, Mao Y, Garg A, Park E, Wu Y, Chow A, Peregrin J, Zhang X. Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development. bioRxiv. 2024 Oct 22; doi: 10.1101/2024.10.20.619055. PubMed PMID: 39484547; PubMed Central PMCID: PMC11527007. eLife 13:RP103615. https://doi.org/10.7554/eLife.103615.1
- Wang Q, Tao C, Wu Y, Anderson KE, Hannan A, Lin CS, Hawkins P, Stephens L, Zhang X. Phospholipase Cγ regulates lacrimal gland branching by competing with PI3K in phosphoinositide metabolism. bioRxiv. 2024 Jul 2;. doi: 10.1101/2024.06.28.601066. (Revision in Cell Reports)
- Wu H, Mao Y, Wang Q, Yu HL, Bouaziz M, Makrides N, Koleske A, Radice G, Zhang X. Abelson kinases regulate FGF signaling independent of Crk phosphorylation to prevent Peters anomaly. bioRxiv. 2024 Oct 26; PubMed Central ID: PMC11526961.
- Hannan A, Wang Q, Wu Y, Qu X, Cardoso W, Zhang X. Crk mediates Csk-Hippo signaling independently of Yap tyrosine phosphorylation in lacrimal gland development. bioRxiv. 2024 Jul 1;. doi: 10.1101/2024.06.27.601065. PubMed PMID: 39005335; PubMed Central PMCID: PMC11244872
- Makrides N#, Wang Q#, Tao C, Schwartz S, Zhang X. Jack of all trades, master of each: the diversity of fibroblast growth factor signalling in eye development. Review. Open Biology. 2022 Jan; 12 (1), 210265 (# Co-first author)
- Wang Q, Tao C, Hannan A, Yoon S, Min X, Peregrin J, Qu X, Li H, Yu H, Zhao J, Zhang X. Lacrimal gland budding requires PI3K-dependent suppression of EGF signaling. Science Advances. 2021 Jun 30;7 (27). Pii:eabf1068
- Balasubramanian R, Min X, Quinn PMJ, Giudice QL, Tao C, Polanco K, Makrides N, Peregrin J, Bouaziz M, Mao Y, Wang Q, da Costa BL, Buenaventura D, Ma L, Tsang SH, Fabre PJ, Zhang X. Phase transition specified by a binary code patterns the vertebrate eye cup. Science Advances. 2021 Nov 12; 7 (46), eabj9846
- Garg A#, Hannan A#, Wang Q#, Makrides N, Zhong J, Li H, Yoon S, Mao Y, Zhang X. Etv transcription factors functionally diverge from their upstream FGF signaling in lens development. Elife. 2020 Feb 11;9. Pii: e51915 (# Co-first author)
- Garg A, Hannan A, Wang Q, Collins T, Teng S, Bansal M, Zhong J, Xu K, Zhang X. FGF-induced Pea3 transcription factors program the genetic landscape for cell fate determination. PloS Genetics. 2018 Sep 6;14(9):e1007660
- Zheng W, Shen F, Hu R, Roy B, Yang J, Wang Q, Zhang F, King JC, Sergi C, Liu SM, Cordat E, Tang J, Chen XZ. Far Upstream Element-Binding Protein 1 Binds the 3’ Untranslated Region of PKD2 and Suppresses Its Translation. Journal of the American Society of Nephrology. 2016 Sep;27(9):2645-57
- Wang Q, Zheng W, Wang Z, Yang J, Hussein S, Tang J, Chen XZ. Filamin-A increases the stability and plasma membrane expression of polycystin-2. PLoS One. 2015 April 10;10(4):e0123018
- Hussein S, Zheng W, Dyte C, Wang Q, Yang J, Zhang F, Tang J, Cao Y, Chen XZ. Acid-induced off-response of PKD2L1 channel in Xenopus oocytes and its regulation by Ca. Scientific Reports. 2015 Oct 27;5:15752
- Schorr S, Klein MC, Gamayun I, Melnyk A, Jung M, Schäuble N, Wang Q, Hemmis B, Bochen F, Greiner M, Lampel P, Urban SK, Hassdenteufel S, Dudek J, Chen XZ, Wagner R, Cavalié A, Zimmermann R. Co-chaperone Specificity in Gating of the Polypeptide Conducting Channel in the Membrane of the Human Endoplasmic Reticulum. Journal of Biological Chemistry. 2015 Jul 24;290(30):18621-35
- Hu Q, Wu Y, Tang J, Zheng W, Wang Q, Nahirney D, Duszyk M, Wang S, Tu JC, Chen XZ. Expression of polycystins and fibrocystin on primary cilia of lung cells. Biochemistry and Cell Biology. 2014 Dec;92(6):547-54
- Yang J, Zheng W, Wang Q, Lara C, Hussein S, Chen XZ. Translational up-regulation of polycystic kidney disease protein PKD2 by endoplasmic reticulum stress. FASEB Journal. 2013 Dec;27(12):4998-5009
- Wang Q, Dai XQ, Li Q, Tuli J, Liang G, Li SS, Chen XZ. Filamin interacts with ENaC and inhibits its channel function. Journal of Biological Chemistry. 2013 Jan 4;288(1):264-73
- Wang Q, Dai XQ, Li Q, Wang Z, Cantero Mdel R, Li S, Shen J, Tu JC, Cantiello H, Chen XZ. Structural interaction and functional regulation of polycystin-2 by filamin. PLoS One. 2012;7(7):e40448
- Yang J, Wang Q, Zheng W, Tuli J, Li Q, Wu Y, Hussein S, Dai XQ, Shafiei S, Li XG, Shen PY, Tu JC, Chen XZ. RACK1 interacts with Pkd2L1 and inhibits its channel function. Journal of Biological Chemistry. 2012 Feb 24; 287: 6551-6561


